Locals donating plasma for COVID-19 treatment protocol, more donations needed
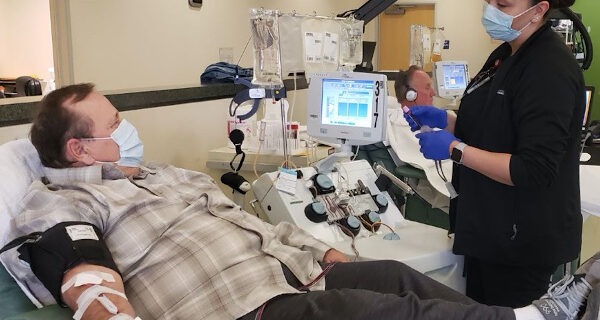
–Paso Robles woman Heather Mikelonis and her husband, who have both recovered from COVID-19, are participating in a convalescent plasma program.
“I heard about donating convalescent plasma last month and thought it sounded like a good idea,” she said. I was put in touch with Dr. Shields Abernathy of Templeton who helped guide me through the process of getting another SARS-CoV-2 test to prove that I am now negative and then get set up with Vitalant to donate plasma.”
“My first call to Dr. Abernathy was on April 13th, and I was able to donate convalescent plasma on April 22nd. The staff at Vitalant are very nice and I found the plasma donation process to be simple and relaxing. It takes about three hours from start to finish and at this point seems to be one of the best ways I can help others. I have another appointment to donate again on May 1.”
Plasma donors needed for COVID-19 treatment protocol
–Vitalant, the nation’s largest, independent nonprofit blood service provider, is asking for those who have recovered from COVID-19 to donate plasma for a Federal Drug Administration approved COVID-19 treatment protocol.
The program has been launched in collaboration with Vitalant, the Mayo Clinic, and the American Red Cross to treat COVID-19 patients with convalescent plasma. The Food and Drug Administration (FDA) has identified and approved convalescent plasma treatment as an investigational therapy and issued “Recommendations for Investigational COVID-19 Convalescent Plasma,” on April 13. An April 24 press release from Vivalant describes the treatment as “a promising new treatment option.” and “currently the only antibody treatment available to COVID-19 patients.”
Those who have completely recovered from COVID-19 may have immune-boosting antibodies in their plasma, called “convalescent plasma,” that could be used to treat critically ill COVID-19 patients. Currently, there are no vaccines or proven treatments for COVID-19 because the virus is so new. Although trials for a vaccine are underway, it is expected to be many months before one is approved. As of April 24, the Mayo Clinic has identified 1726 patients who have been infused with the plasma. Nationally, 1978 sites, 3251 physicians and 4524 patients have participated.
“Blood transfusions help patients for a variety of reasons—from surviving trauma, to undergoing chemotherapy to receiving an organ transplant,” said Cliff Numark, Vitalant’s chief of marketing. “A convalescent plasma transfusion holds the additional promise of helping a critically ill COVID-19 patient turn the corner and head toward a positive outcome.”
Vitalant encourages people who have recovered from COVID-19 (or suspected they had it), to fill out a prescreening form to begin the process for determining their eligibility or call 866-CV-PLSMA (866-287-5762). Potential donors will then be contacted by Vitalant.
Eligibility criteria are:
- Prior diagnosis of COVID-19, documented by a laboratory test
- Complete resolution of symptoms for at least 14 days
- Meet all other current FDA donor eligibility requirements to donate plasma
In addition, potential convalescent plasma donors can be referred to Vitalant by their physician, clinic, hospital or local public health departments.
Locally, those interested in donating blood plasma can contact Dr. Shields B. Abernathy at his Templeton office (805) 434-1000.
FDA announces two investigative therapies
In an April 3 press release, the FDA announced two investigative therapies derived from human blood, the convalescent plasma and hyperimmune globulin. Both are antibody-rich blood products made from blood donated by people who have recovered from the virus. “The products can be administered to individuals diagnosed with COVID-19. There are some limited data to suggest that convalescent plasma and hyperimmune globulin may have benefit in the COVID-19 illness. This is why evaluation of these therapies in the context of a clinical trial and expanded access program is so important.”
On April 6 the Swedish company Octapharma announced an alliance with Takeda (Japan), CSL Limited (Australia) and United States companies BPL Theraputics, Biotest and LFB, to develop a potential plasma-derived hyperimmune immunoglobulin therapy for treating COVID-19.
“The alliance will immediately begin with the investigational development of one, unbranded anti-SARS-CoV-2 polyclonal hyperimmune immunoglobulin medicine with the potential to treat individuals with serious complications from COVID-19.”
Comments
Scott Brennan is the publisher of this website and founder of Access Publishing. Connect with him on Google+, Twitter, LinkedIn, or follow his blog.










